The potential vaccine may offer a preventative treatment for some of the poorest people on the planet, with rising cases attributed to deforestation and urbanisation.
Leishmaniasis is a rare disease in the Western world, but it affects millions of people across the world.
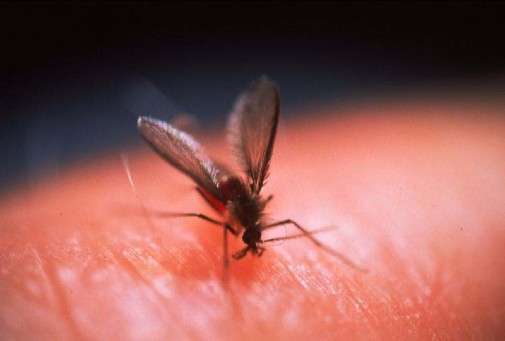
The serious disease is developed from the bite of an affected female sandfly, specifically by the protozoan Leishmania parasites, transmitted when the fly feeds on a host. This disease is particularly prominent in the tropics, subtropics, and southern Europe where the flies are most accustomed to the climate.
The parasites responsible for the disease are called Leishmania, named after British pathologist William B. Leishman. When infected female sandflies bite a host the disease is transmitted from the fly to the human from the transfer of bodily fluids. The flies bite hosts as they are a food source, needed for them to live and produce eggs.
The form of leishmaniasis developed by the host depends on factors including the characteristics of the parasite and sandfly species, the ecology of the transmission site, and even whether the host had previously been infected.
If scientists were able to vaccinate for this disease it would reduce the number of cases developed, the severity of those cases and ultimately death rates.
Up to a million new cases of Leishmaniasis occur annually, often devastating communities due to the ferocity of the disease, impacting many parts of the body. There are three established types:
- Visceral leishmaniasis: the most severe form, with over 95% of cases proving fatal to the patient when untreated. Symptoms include but are not limited to: enlargement of the liver, chronic anaemia and extreme weight loss. This form is highly contagious with a continuous high mortality rate.
- Cutaneous leishmaniasis: the most common form of leishmaniasis causing permanent skin damage from lesions and ulcers. Patients are often left with extremely visible scarring and disability creating enormous stigma and prejudice within communities.
- Mucocutaneous leishmaniasis: this form destroys (fully or partially) the mucous membranes of the nose, mouth and throat. This form is extremely rare and often occurs months after the exposure to the parasite.
Professor Paul Kaye, from Hull York Medical School, was the principal investigator on the Wellcome Trust Translation Award that funded the development of the vaccine. The existing drugs used to prevent the disease have considerable side-effects and are incredibly difficult to administer.
Kaye said: “we have always thought that vaccines should be our greatest weapon against the different forms of leishmaniasis, but it has been a long journey to develop vaccines for testing in the clinic”. According to the Wellcome Trust, the research, development and safe testing of a vaccine often takes ten years costing $500 million.
This is distinctly different from the development of the Covid-19 vaccine, which broke the record amount of time for creating a vaccine (which was four years for the mumps vaccine).
The quick development of the Covid-19 vaccine was partially due to previous research done on the coronavirus family. There are hundreds of coronaviruses some of which can cause common cold, some of which sparked the SARS epidemic, and some of which caused the devastation of MERS (Middle East Respiratory Syndrome).
This potential leishmaniasis vaccine is similar in design to the AstraZeneca Covid-19 vaccine, using a non-replicating virus to initiate coding for the Leishmania proteins by a set of genes. All of this is done aiming to prevent people from developing the disease.
Kaye described the results as “very encouraging, showing that the vaccine we have developed is safe and immunogenic in patients. It is now important to test this vaccine as a therapy in different forms of leishmaniasis where drugs are poorly effective, and to see if it can prevent the spread of the disease”.
Vaccines have dominated the news for the past few months, with pressure on researchers around the globe to obtain a safe and effective vaccine as quickly as possible to treat the coronavirus pandemic. Yet vaccines are sometimes misunderstood. A vaccine provides immunity for a particular disease, so a vaccine to treat the common cold would not treat something like the coronavirus.
Vaccines typically contain a part of a disease, but it is disabled completely so it would be unable to replicate within the host. Regardless of if a virus is active or weakened or even disabled completely, it will have surface proteins on the virus. These proteins are key to the production of a vaccine. When a virus enters the body, the body produces antibodies that can disarm the virus. Each type of surface protein is different on each virus, meaning that for each new viral infection the body will need to produce new antibiotics.
When you get a vaccine, regardless of if it is for the flu or Covid-19, it would be impossible for that virus to replicate within your body. This means after vaccination, if you were to come into contact with the virus, your chances of getting seriously infected and infecting others would be significantly reduced.
The implementation of a safe vaccine programme is a very effective way to help treat communities against particular diseases. The alternative method of exposing a population to a disease without appropriate protection would put them at considerable risk.
The development of this vaccine is particularly significant because currently there is no vaccine to prevent Leishmaniasis, despite the number of annual cases and the severity of the disease.
The current trials underway are testing the vaccine to see if it can treat rather than prevent the disease. The results so far show that it has been able to safely stimulate an immune response for patients with chronic skin form of leishmania.
A second trial of the vaccine has been launched, which will determine whether the vaccine alone can treat patients after exposure to this virus without the need for further medical intervention. Following this research, the researchers will test the vaccine on healthy volunteers to determine whether it can immunise patients as well as treating symptoms post-exposure.